Chemical Composition of Red 40
Is red food coloring red 40 – Red 40, also known as Allura Red AC, is a synthetic azo dye widely used as a food coloring. Understanding its chemical structure, synthesis, properties, and comparison to other colorants is crucial for assessing its safety and application in the food industry.
Chemical Structure of Red 40
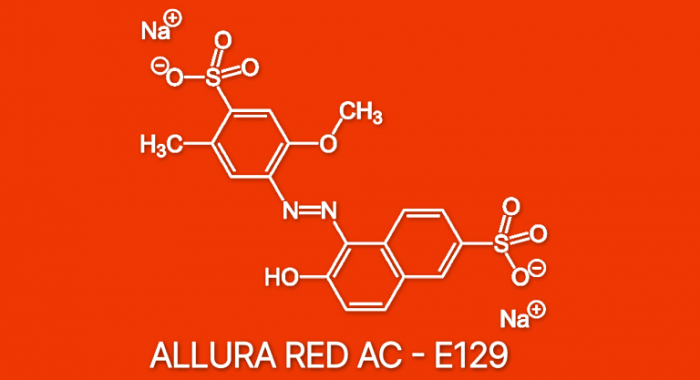
Red 40’s chemical structure is a disodium salt of a sulfonated azo dye. Its IUPAC name is disodium 6-hydroxy-5-((2-methoxy-4-sulfophenyl)azo)-2-naphthalenesulfonate. The molecule consists of two aromatic rings (a naphthalene ring and a benzene ring) linked by an azo group (-N=N-), a characteristic feature of azo dyes. Several substituent groups, including hydroxyl (-OH), methoxy (-OCH 3), and sulfonate (-SO 3Na) groups, are attached to the rings, influencing its color and properties.
So, you’re wondering if that vibrant red in your cake is actually Red 40? It’s a common question! Many brands, including the intensely pigmented colors you get from betty crocker gel food coloring , often use Red 40, but always check the ingredient list to be sure. Ultimately, knowing what’s in your food coloring helps you make informed choices about what you bake and eat.
The presence of the sulfonate groups makes it highly water-soluble.
Synthesis of Red 40
The synthesis of Red 40 involves a diazotization reaction followed by a coupling reaction. First, 2-methoxy-4-sulfanilic acid is diazotized with sodium nitrite (NaNO 2) and hydrochloric acid (HCl) to form a diazonium salt. This diazonium salt is then coupled with 6-hydroxy-2-naphthalenesulfonic acid, resulting in the formation of Red 40. The process requires careful control of reaction conditions, including temperature and pH, to ensure high yield and purity.
Properties of Red 40
Red 40 is a bright red powder that is readily soluble in water. Its solubility is significantly influenced by the presence of the sulfonate groups, which contribute to its ionic character. It is relatively stable under normal conditions, although its color intensity may be affected by pH changes, light exposure, and high temperatures. Red 40 exhibits maximum absorbance at around 504 nm, contributing to its vibrant red color.
It is also relatively stable in acidic and slightly alkaline conditions.
Comparison of Red 40 to Other Red Food Colorings
Red 40 differs chemically from other red food colorings. For instance, Carmine (derived from insects) is a natural dye with a different chemical structure, while other synthetic azo dyes, such as Amaranth, have variations in their substituent groups, leading to subtle differences in color and properties. These variations impact their stability and potential interactions with other food components.
Comparison of Red 40 to Natural Red Color Sources
| Property | Red 40 | Beet Juice | Paprika |
|---|---|---|---|
| Source | Synthetic | Natural (Beetroot) | Natural (Paprika pepper) |
| Chemical Composition | Disodium 6-hydroxy-5-((2-methoxy-4-sulfophenyl)azo)-2-naphthalenesulfonate | Betalains (Betanin, Isobetanin) | Capsanthin, Capsorubin, other carotenoids |
| Solubility | High in water | Moderate in water | Low in water, soluble in oils |
| Stability | Relatively stable | Sensitive to heat, light, and pH | Sensitive to heat, light, and oxidation |
Red 40’s Appearance and Perception

Red 40, also known as Allura Red AC, owes its vibrant color to its interaction with visible light. Its chemical structure allows it to absorb certain wavelengths of light while reflecting others, specifically in the red portion of the electromagnetic spectrum. This selective absorption and reflection are what our eyes perceive as the characteristic red hue.
Red 40’s Light Interaction and Color Perception
The color we see is determined by the wavelengths of light that are not absorbed by the molecule. Red 40’s structure selectively absorbs light in the blue-green region of the spectrum, leading to the reflection of red light which is then detected by our eyes. The intensity of the red color depends on the concentration of Red 40; higher concentrations absorb more light, resulting in a more intense red.
Factors Influencing Red 40’s Shade, Is red food coloring red 40
Several factors can influence the precise shade of red produced by Red 40. The pH of the solution plays a significant role. Changes in pH can alter the molecule’s structure, slightly shifting its absorption and reflection properties, leading to variations in the perceived color. For example, a slightly more orange or purplish hue might be observed at different pH levels.
Concentration is another key factor; higher concentrations yield a more intense, darker red, while lower concentrations result in a paler, lighter red.
Red 40 in Common Foods and its Color Contribution
Red 40 is widely used in various food products to enhance their visual appeal. In beverages like fruit-flavored drinks and sports drinks, it contributes to the characteristic bright red color. In candies, jellies, and baked goods, it provides vibrant red hues that enhance the product’s attractiveness. The color intensity in these products is often carefully controlled through precise concentrations of Red 40 to achieve the desired visual effect.
For example, a strawberry-flavored yogurt might use a lower concentration for a pastel pink, while a cherry-flavored candy might use a higher concentration for a deep, intense red.
Variations in Red 40 Color Across Manufacturers and Batches
While Red 40 is a standardized food dye, minor variations in color can occur between different manufacturers or even different batches from the same manufacturer. These variations are typically subtle and usually fall within acceptable industry tolerances. They can arise from slight differences in the manufacturing process, the purity of starting materials, or even variations in storage conditions. However, these differences are generally insignificant enough to not significantly impact the overall appearance of the food product.
Visual Representation of Red 40 Concentration and Color Intensity
A visual representation could be a series of six small square containers filled with water. Each container would contain a progressively increasing concentration of Red 40, starting from a very dilute solution (almost clear) to a highly concentrated solution (a very deep red). The containers would be clearly labeled with their corresponding Red 40 concentration (e.g., 0.01%, 0.05%, 0.1%, 0.2%, 0.5%, 1%).
This would visually demonstrate the relationship between concentration and color intensity, showing how a small increase in concentration leads to a noticeable change in the shade and intensity of the red color. The containers could be arranged in a row or in a gradient pattern, further highlighting the visual differences.
FAQ Summary: Is Red Food Coloring Red 40
Is Red 40 vegan?
Generally, yes. Red 40 is typically derived from petroleum, making it suitable for vegan diets. However, always check product labels to ensure no animal-derived ingredients are present in the specific product.
Does Red 40 cause hyperactivity in children?
This is a common misconception. While some studies have suggested a link, extensive research, including meta-analyses, hasn’t definitively established a causal relationship between Red 40 and hyperactivity.
Where can I find Red 40’s chemical formula?
You can easily find the chemical formula (Allura Red AC) and its structure through a quick online search. Many scientific databases and websites provide detailed information on food additives.
Are there any long-term health effects associated with Red 40 consumption?
Current research hasn’t established any definitive long-term health effects from consuming Red 40 within acceptable daily intake levels. However, ongoing research continues to monitor its safety profile.
